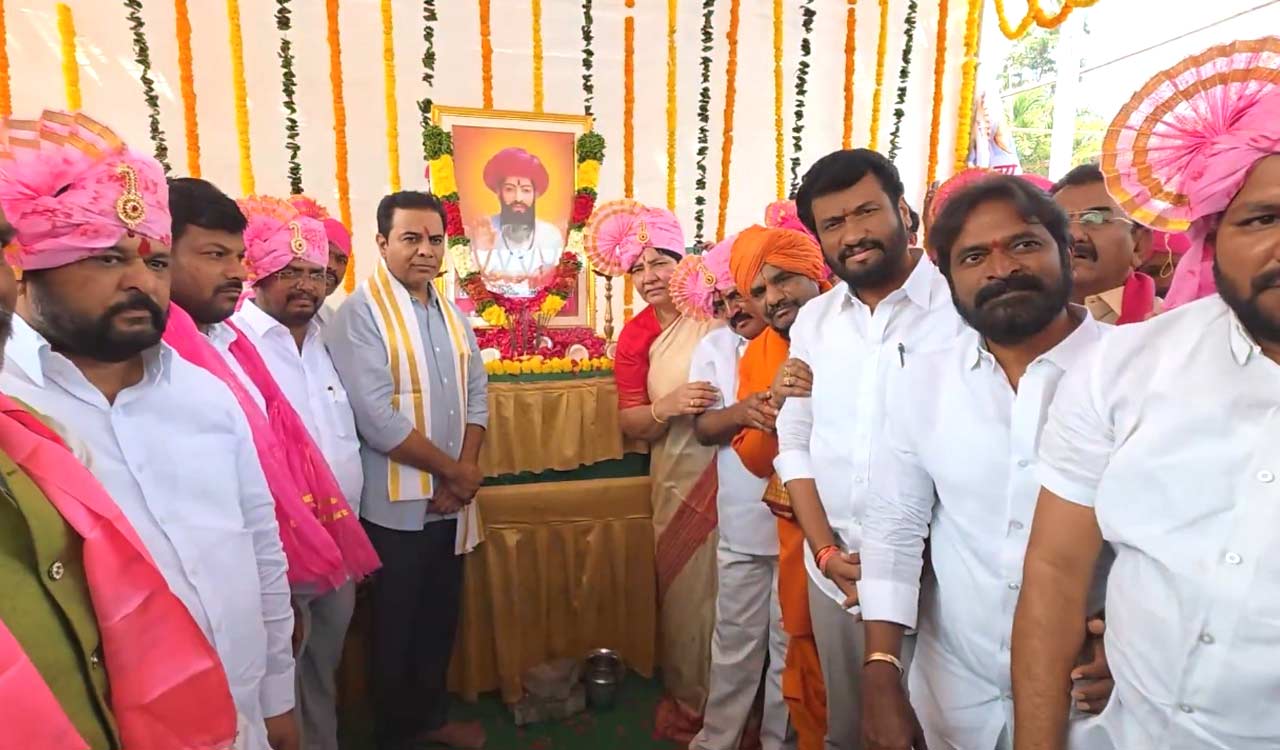
BRS working president KT Rama Rao paid tributes to Sant Sevalal Maharaj and highlighted tribal welfare initiatives undertaken during the BRS regime, while alleging attempts by the Centre and State governments to weaken Singareni Collieries.
Published Date – 15 February 2026, 02:18 PM
Hyderabad: BRS working president KT Rama Rao paid floral tributes to Sant Sevalal Maharaj during the latter’s birth anniversary celebrations at Telangana Bhavan on Sunday. He credited BRS president and former Chief Minister K Chandrashekhar Rao with strengthening tribal self-respect during his tenure, on the path guided by Sant Sevalal Maharaj, the spiritual leader of the Banjara community.
Speaking on the occasion, Rama Rao recalled that the previous BRS government developed tribal hamlets and created 3,146 new gram panchayats, enabling tribal representatives to become sarpanchs. He stated that drinking water facilities were extended to tribal habitations and land titles for 4.5 lakh acres were cleared in a single day.
The BRS working president pointed to hundreds of residential Gurukul schools established for tribal students and enhanced job reservations for ST under Chandrashekhar Rao’s leadership. He reminded that the CMST Entrepreneurship Programme was introduced to support tribal entrepreneurs and subsidies worth lakhs of rupees were provided during the BRS regime.
He added that overseas scholarships were granted to Banjara students and Banjara Bhavans were sanctioned and constructed in districts across the State as well as in Banjara Hills of Hyderabad. He assured continued support to the community.
Responding to a media query, Rama Rao said both the Centre and the State governments were attempting to weaken Singareni Collieries.
“Hence, we offered to support CPI to win the Kothagudem Municipal Corporation to safeguard Singareni and its workers. CPI is an alliance partner of the Congress, and they might to join hands. But we will continue our fight for Singareni,” he asserted, adding that the ruling Congress was harassing elected BRS councillors and misusing police machinery to win civic bodies during the election of municipal chairpersons and municipal corporation Mayors to be held on Monday.